
The particles that make up an atom are called subatomic particles ( sub- means “smaller size”). 2.10).ĭetermine how charged matter interacts. Common examples of static electricity are when someone gets a shock when reaching for a doorknob or when a child’s hair is raised when going down a plastic slide (Fig. The electrostatic forces that hold atoms together in molecules are the same type of forces that cause static electricity. 2.9).Įlectrostatic forces hold atoms in molecules.
Methane (CH 4), a common greenhouse gas, has five atoms, one of carbon (C) and four of hydrogen (H, see Fig. For example, water (H 2O) has three atoms, two hydrogen (H) atoms and one oxygen (O) atom. Molecules of compounds have atoms of two or more different elements. All elemental molecules are made of atoms of a single element. Another form of oxygen, ozone (O 3), has three atoms, and sulfur (S 8) has eight atoms. Hydrogen (H 2), oxygen (O 2), and chlorine (Cl 2) molecules, for example, each contains two atoms. Other elements contain two or more atoms in their molecular form (Fig. 2.8) is an example of a monatomic element. Some elements are monatomic, meaning they are made of a single ( mon-) atom ( -atomic) in their molecular form. A hundred million (100,000,000) hydrogen atoms put side-by-side is only as long as one centimeter! The simplest structural unit of an element is an atom. (ii) Isotope of uranium ( 235U) is used as a fuel in nuclear reactors.The properties of elements and compounds are determined by their structures. (i) Isotope of cobalt, ( Co), is used in the treatment of cancer. For example, Ne has atomic number as 10 and sodium has atomic number as 11 but both of these have mass numbers as 22. (iv) Isobars are such atoms which have same mass number but different atomic numbers. For example, chlorine has two isotopes with atomic number 17 but mass numbers as 35 and 37. (iii) Isotopes are atoms of the same element thus having same atomic number but different mass number. For example there are 6 protons and 6 neutrons in the nucleus of carbon, so its mass number is 12.

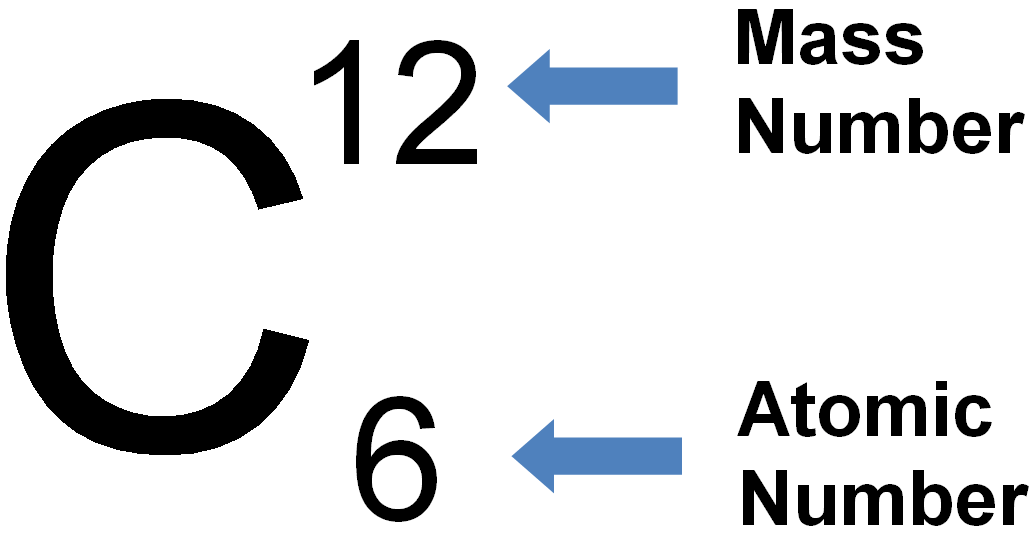
(ii) Mass number is defined as the sum of the total number of protons and neutrons present in the nucleus of an atom. All atoms are characterized by their atomic numbers. For example, there are 6 protons in carbon, so the atomic number of carbon is 6. (i) Atomic number is defined as number of protons present in the nucleus of an atom. There is a loss or gain in energy of electron when it moves from one orbit to the other.
/atomic-structure-artwork-549603139-57fe40e75f9b586c3537ebf4.jpg)
To explain stability of atom and atomic spectra, Bohr suggested that electrons are moving round the nucleus in orbits which have fixed energy shells. This will lead to loss of energy of the moving electron and ultimately giving unstable model of atom. Since charged bodies moving in circular motion emit radiations. Iii)A very small fraction of (a)-particles were deflected by 180 0, indicating that all the positive charge and mass of the gold atom were concentrated in a very small volume with in the atom.ģ.)Neils Bohr. Ii)Very few particles were deflected from their path, indicating that the positive charge of the atom occupies very little space. I)Most of the space inside the atom is empty because most of the (a)-particles passed through the gold foil without getting deflected.
On the basis of above experiment Rutherford concluded that: So, the atom as a whole is electrically neutral.Ģ.)Rutherford designed a model in which fast moving alpha (a)-particles were made to fall on a thin gold foil. Ii)The negative and positive charges are equal in magnitude. I)An atom consists of a positively charged sphere and the electrons are embedded in it. On the base of this model Thomson proposed that: In which the positive charge in atom is spread all over like the red edible part of the watermelon, while the electrons are studded in the positively charged sphere, like the seeds in the watermelon. 1).Thomson proposed the model of an atom to be similar to that of a watermelon.


 0 kommentar(er)
0 kommentar(er)
